intro
This is a page that is geared more towards those who might be thinking of making cold pour soap. All are welcome! It’s a great eye opener for those who don’t know how soap really works… like, I always wondered how and why soap cleans and disinfects. The section that describes “micelles” takes care of this.
Get ready, after this you’re going to need a bar of my soap to wash all the science off of you, you’re about to get neck deep in it!

lye safety
When discussing how soap is made, Lye safety has to come first before we do anything. Lye is also commonly known as “caustic soda” (“caustic” – able to burn or corrode organic tissue by chemical action). >The “soda” part of the name comes from its historical connection to soda ash (sodium carbonate).
Unlike acids, which tend to form a protective layer (eschar) that limits further penetration, alkalis cause liquefactive necrosis, which dissolves tissues and allows the alkali to penetrate deeper and continue causing damage. This explains why alkali burns are often more severe and can cause significant tissue damage.
Important safety note: Mixing sodium hydroxide with water is an exothermic reaction, meaning it generates heat. Always add NaOH to water slowly while stirring to minimize heat generation and prevent splashing. I recommend having a mix of water “states”, that is, part ice and part water, which will help contain fuming and help the minimize the generation of heat from the exothermic reaction.
Never add water to lye, as this can cause a violent exothermic reaction and release harmful fumes.
Instead, always add lye slowly to water while stirring to control the reaction and heat generation.
The heat generated during the dissolution of lye in water can be substantial, reaching temperatures up to 200°F (93°C).
A clever mnemonic I have heard is: “Add your lye to the water like you otta, add the water to your lye and you dye”.
Again, lye safety, always wear gloves and eye protection when working with lye. Do not take off your gloves after mixing the lye into your oils! You will handle the soap with bare hands and get a chemical burn. Keep the gloves on! please & thank you 🙂
First Aid for alkali burns:
1. Flush with water, irrigate the affected area with large amounts of cool or lukewarm running water for at least 20 minutes. This helps to dilute and wash away the alkali.
2. Do not attempt to neutralize with acid, avoid using vinegar or other acidic solutions to neutralize alkali burns, as this can generate heat and make the burn worse!
what composes fats, oils, and butters?
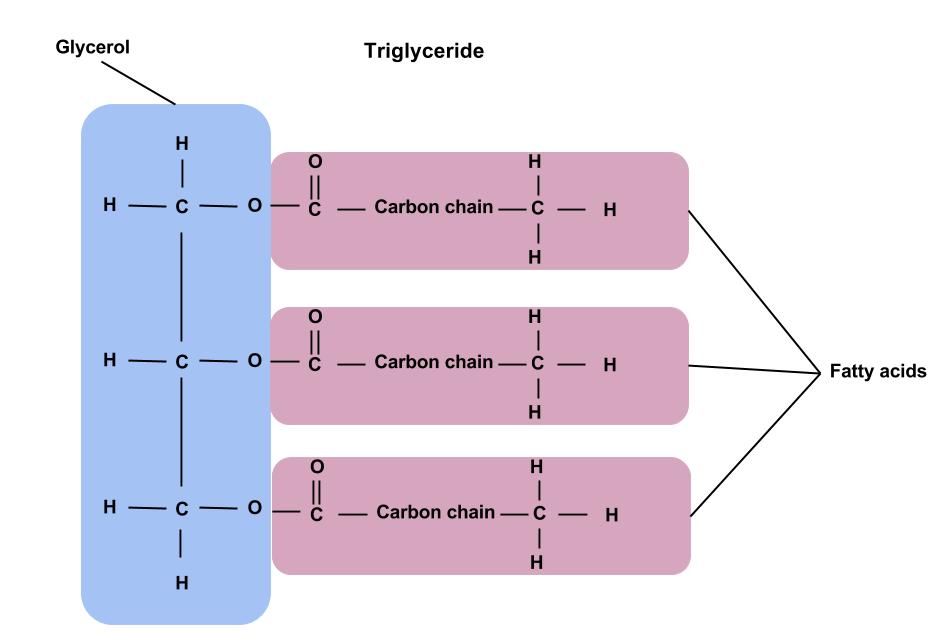
Fats and oils (“lipids“) contain polymers known as triglycerides. Polymers are larger units composed of smaller units known as “monomers“, and fatty acids are a type of monomer! So is glycerol. A triglyceride is a glycerol molecule with three fatty acid chains attached to it. Wait, what do you mean “chain”? Chains can be saturated (all single bonds) or unsaturated (containing one or more double bonds). The length of the chain and the presence/absence of double bonds determine the type of fatty acid and its properties. Wait, what is a bond? Carbon atoms primarily form covalent bonds with other carbon atoms. (Although fatty acid chains also contain Oxygen and Hydrogen). These covalent bonds are formed by the sharing of electron pairs between the carbon atoms, rather than a complete transfer of electrons (as in ionic bonding).
fatty acid chains
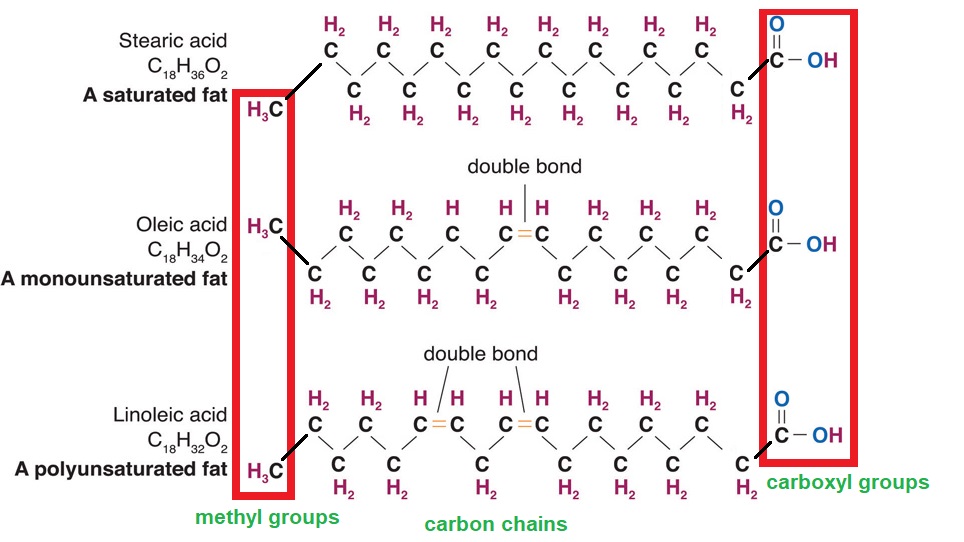
It’s all about the Chain. Fatty acids have different chain lengths and different compositions. Oils that have fatty acids with chain lengths between four and twenty-four carbons and most of them contain an even number of carbon atoms. When the carbon chain length is shorter, the melting point of the fatty acid becomes lower, and the fatty acid becomes more liquid.
Fatty acid chains are held together by carbon atoms that attach to each other and to hydrogen atoms. The term saturation refers to whether or not a fatty acid chain is filled (or “saturated”) to capacity with hydrogen atoms. If each available carbon bond holds a hydrogen atom, we call this a saturated fatty acid chain. All carbon atoms in such a fatty acid chain are bonded with single bonds. Sometimes the chain has a place where hydrogen atoms are missing. This is referred to as the point of unsaturation.
When one or more bonds between carbon atoms are a double bond (C=C), that fatty acid is called an unsaturated fatty acid, as it has one or more points of unsaturation. Any fatty acid that has only one double bond is a monounsaturated fatty acid, an example of which is olive oil (75 percent of its fat is monounsaturated). A polyunsaturated fatty acid is a fatty acid with two or more double bonds or two or more points of unsaturation. Soybean oil contains high amounts of polyunsaturated fatty acids. Both monounsaturated fats and polyunsaturated fats provide nutrition that is essential for normal cell development and healthy skin.
Oils and butters that have a high percentage of saturated fatty acids tend to be solid at room temperature. Coconut oil is a saturated fat. It contains approximately 90% saturated fatty acids, which are a type of fat that is solid at room temperature. It is primarily composed of saturated fats, particularly medium-chain fatty acids (MCFAs) like lauric acid. Oils rich in unsaturated fatty acids, such as olive oil (oleic acid, an eighteen-carbon unsaturated fatty acid, is a major component) tend to be liquid at room temperature. Flaxseed oil is rich in alpha-linolenic acid, which is an unsaturated fatty acid and becomes a thin liquid at room temperature.
Shea butter primarily contains long-chain fatty acids, specifically those with 18 carbon atoms. The main fatty acids in shea butter are stearic acid (C18:0) and oleic acid (C18:1), along with smaller amounts of linoleic acid (C18:2). The numbers refer to carbon molecules to the left of the colon, and number of double bonds to the right of the colon.
Castor oil is primarily composed of ricinoleic acid (18:1), a monounsaturated, 18-carbon fatty acid. This means the main fatty acid in castor oil has a chain length of 18 carbons. It’s unusual among fatty acids due to a hydroxyl group on the 12th carbon atom.
Short-chain fatty acids are found in plant sources like flax, chia, hemp, canola oil, and walnuts.
Medium Chain fatty acids like coconut oil, A primary source of MCTs, with varying concentrations of capric, caprylic, and lauric acids.
Palm kernel oil, another natural source of MCTs. Caproic acid (C6:0), caprylic acid (C8:0), capric acid (C10:0), lauric acid (C12:0). they are a type of saturated fats.
Long chain fatty acids include coconut oil, palm kernel oil, shea oil, cocoa butter, olive oil, soybean oil, and many other vegetable oils contain significant amounts of LCFAs.
LCFAs are also found in animal fats like butter, lard, and tallow, as well as in foods of animal origin like fatty fish (salmon, herring, trout). Avocado, nuts, and seeds are good sources of LCFAs. Avocados contain both saturated and unsaturated fats, approximately 75% of the fat in an avocado is considered good fat, including monounsaturated and polyunsaturated fats.
Knowing the connection between chain length, degree of saturation, and the state of the fatty acid (solid or liquid) is critical for the formulation of your soap recipe.
lye and pH
Lye is the compound sodium hydroxide (NaOH).
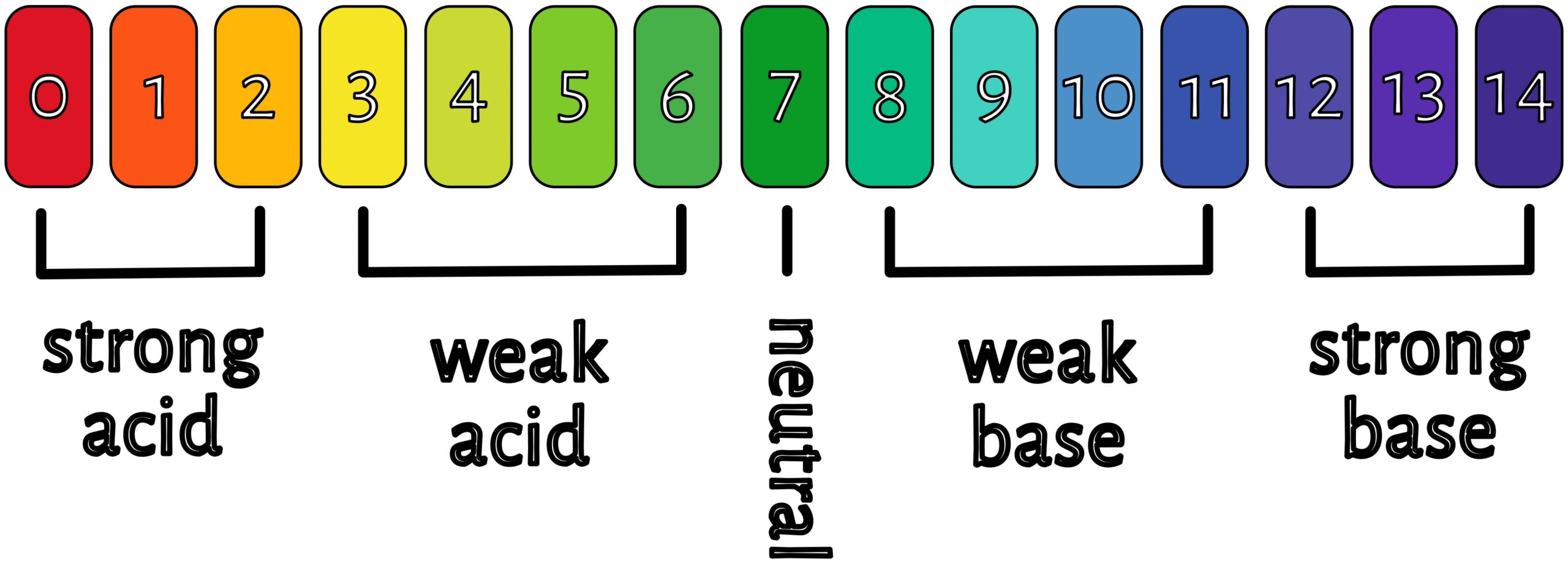
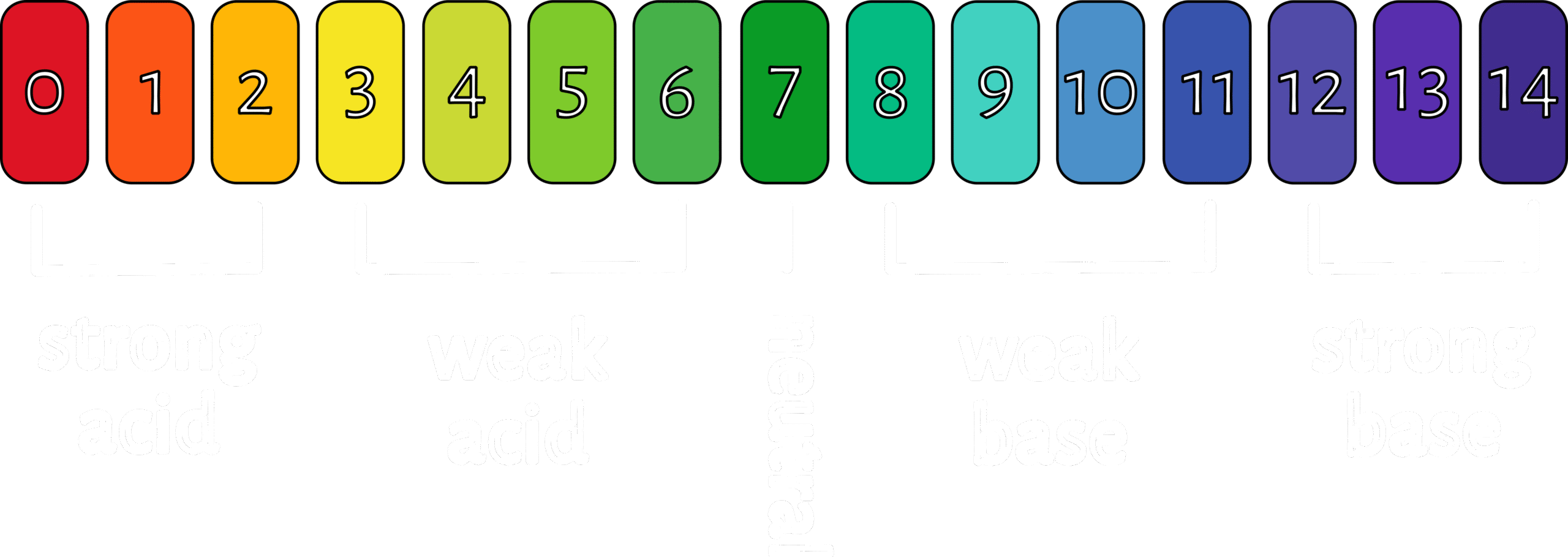
pH (potential for hydrogen) measures the concentration of hydrogen ions (H+) in a solution. A higher concentration of hydrogen ions means a solution is more acidic, and a lower concentration means it’s more basic (alkaline).
Lye functions as an alkali, reacting with fats or oils to produce soap and glycerin. The pH of sodium hydroxide is typically around 13-14.
The pH of soap typically falls within a basic range, usually between 8 and 10, with some soaps even reaching 11. This means soap is alkaline rather than neutral or acidic. The pH of soap can affect its suitability for different skin types, with more alkaline soaps potentially being harsher for sensitive skin. Different oils and fats used in soap making can affect the final pH as well as the amount and type of lye used to saponify the fats play a crucial role in determining the pH.
Now, Sodium hydroxide, also known as lye, needs to be dissolved in a liquid to dissociate into sodium ions (Na+) and hydroxide ions (OH-). Hydrogen ions (H⁺) and hydroxide ions (OH⁻) are fundamental to understanding acids and bases, particularly in aqueous solutions. The hydroxide ions are crucial for the saponification reaction to occur. They are the active agents that interact with the fats/oils to break them down into soap and glycerin. While water doesn’t appear directly in the final chemical equation of saponification, it acts as a solvent, releasing hydroxide ions from the compound NaOH allowing it to interact with the oils and fats effectively.
It’s also a good idea to mix your lye solutions beforehand, so they are at least room temperature the day of. This is because increasing temperature provides the reactant molecules with the necessary energy to collide effectively and drive the saponification reaction forward at a higher rate.
saponification
The process of making soap is a chemical reaction known as “saponification“. Soaponification would have been better… just saying…
Triglyceride (Fat/Oil) + Sodium Hydroxide (NaOH) → Glycerol + Soap (Sodium Salt of Fatty Acid)
Saponification involves the breaking of bonds within a single reactant (the triglyceride).
It is a decomposition reaction. It involves the breakdown of a polymer (triglyceride) into monomers glycerol and fatty acid salts (soap). The breakdown known as hydrolysis (water is added) occurs with a strong base like sodium hydroxide breaking the ester bonds in the triglyceride.
As I understand it, soap is a completely different compound created from a hydrolysis chemical reaction. This is NOT an emulsion of oil and water. Although, the end product of the reaction, which is soap, acts as an emulsifier of water and oil by acting as a surfactant.
Now I know what you’re thinking…
Emulsifiers are substances that allow oil and water to mix and remain mixed, and sodium hydroxide’s role in soap making is to break down fats and oils, not to stabilize them in an emulsion.
Emulsification is not a part of the saponification reaction itself, but it is a key mechanism through which the soap produced by saponification performs its cleaning function.
The fatty acids created during saponification act as surfactants, which allow oil and water to mix.
how does soap clean and disinfect?
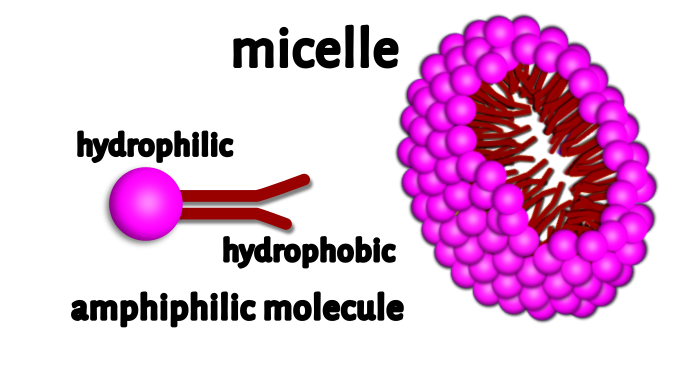
A micelle is a wheel-like structure formed by the circle of soap molecules around a dirt or oil droplet. The molecules of soap of sodium salts are made of long chain carboxylic acids. The ionic end of the salts of soap dissolves in the water of the soap solution, while the carbon chain dissolves in oil which is present in the dirt, forming structures called micelles. Micelles are formed by amphiphilic molecules which are present in the salt of the soap. The structures of the micelles contain hydrophilic (water loving) and hydrophobic (water hating) ends. They surround dirt and float away in the water rinse. They break down dirt and pathogens.
Here’s how soap micelles break down pathogens:
Soap molecule structure: Each soap molecule has a hydrophilic (water loving) head and a hydrophobic (water hating) tail.
Interaction with fats and oils: The hydrophobic tail of the soap molecule is attracted to fats and oils. Many bacteria and viruses, including coronaviruses, are enclosed within a fatty lipid membrane.
Disruption of lipid membranes: When soap encounters these pathogens, the hydrophobic tails of the soap molecules wedge themselves into the lipid membranes of the bacteria and viruses, effectively prying them apart.
Micelle formation: As the soap molecules interact with the lipids, they form micelles, which are tiny spherical clusters. The hydrophobic tails of the soap molecules surround the fragments of the disrupted lipid membrane, with the hydrophilic heads facing outwards towards the water.
Encapsulation and removal: The formed micelles encapsulate the disrupted lipid membrane fragments and any associated genetic material or proteins, making them soluble in water.
Washing away: When you rinse with water, the hydrophilic heads of the micelles are attracted to the water molecules, and the entire structure, including the trapped pathogen components, is washed away.
Essentially, the soap micelles act as molecular “crowbars”, destabilizing the lipid membranes of the pathogens and then encapsulating the resulting fragments so they can be effectively rinsed away with water. This action inactivates the pathogens and removes them from surfaces.
Soap molecules dislodge dirt, oil, and grease particles, and the disease-causing germs they carry, from your hands, one micelle at a time.
finally
So that was a lot to digest! Now you know how our stomachs feel…
All of the above being said, how does this effect the soap making process? The amount of water in a cold process soap recipe significantly affects the soap’s characteristics. Water quantity influences trace time, gel phase, saponification, and ultimately, the final soap’s hardness, cure time, and appearance.
The temperature of your lye solution and oils will greatly effect the speed of the “trace”. Lye and oil being an exothermic reaction means that the hotter your lye solution (and oils) the quicker the reaction. Higher temperatures will shift the equilibrium of exothermic reactions towards the reactants, favoring their formation. This is because increasing the temperature adds heat, which is considered a product in exothermic reactions. According to Le Chatelier’s principle, the system will counteract this stress by favoring the reverse reaction, consuming the added heat and shifting the equilibrium to the left. In the saponification reaction, the primary reactants are fats or oils (triglycerides) and a strong base, typically sodium or potassium hydroxide (lye). These reactants are also sometimes referred to as “esters” in organic chemistry. The reaction also requires water for the hydrolysis process.
Some oils have longer fatty acid chains (shea butter, coconut oil) and therefore have a higher melting point (the temperature at which time they become liquid). Some oils have shorter fatty acid chains (olive oil, cator oil) and therefore are liquid at much lower temperatures. Knowing this will help you create a recipe that you can keep in a state that is workable for soap molding.
Since we’re talking so much science, though it is true that water in its solid form (ice) will increase by about 9% volume, the state’s mass is still the same. In other words, 1 gram of water is one gram of water whether frozen, liquid, or steam.
lye safety
When discussing how soap is made, Lye safety has to come first before we do anything. Lye is also commonly known as “caustic soda” (“caustic” – able to burn or corrode organic tissue by chemical action). >The “soda” part of the name comes from its historical connection to soda ash (sodium carbonate).
Unlike acids, which tend to form a protective layer (eschar) that limits further penetration, alkalis cause liquefactive necrosis, which dissolves tissues and allows the alkali to penetrate deeper and continue causing damage. This explains why alkali burns are often more severe and can cause significant tissue damage.
Important safety note: Mixing sodium hydroxide with water is an exothermic reaction, meaning it generates heat. Always add NaOH to water slowly while stirring to minimize heat generation and prevent splashing. I recommend having a mix of water “states”, that is, part ice and part water, which will help contain fuming and help the minimize the generation of heat from the exothermic reaction.
Never add water to lye, as this can cause a violent exothermic reaction and release harmful fumes.
Instead, always add lye slowly to water while stirring to control the reaction and heat generation.
The heat generated during the dissolution of lye in water can be substantial, reaching temperatures up to 200°F (93°C).
A clever mnemonic I have heard is: “Add your lye to the water like you otta, add the water to your lye and you dye”.
Again, lye safety, always wear gloves and eye protection when working with lye. Do not take off your gloves after mixing the lye into your oils! You will handle the soap with bare hands and get a chemical burn. Keep the gloves on! please & thank you 🙂
First Aid for alkali burns:
1. Flush with water, irrigate the affected area with large amounts of cool or lukewarm running water for at least 20 minutes. This helps to dilute and wash away the alkali.
2. Do not attempt to neutralize with acid, avoid using vinegar or other acidic solutions to neutralize alkali burns, as this can generate heat and make the burn worse!
what composes fats, oils, and butters?

Fats and oils (“lipids“) contain polymers known as triglycerides. Polymers are larger units composed of smaller units known as “monomers“, and fatty acids are a type of monomer! So is glycerol. A triglyceride is a glycerol molecule with three fatty acid chains attached to it. Wait, what do you mean “chain”? Chains can be saturated (all single bonds) or unsaturated (containing one or more double bonds). The length of the chain and the presence/absence of double bonds determine the type of fatty acid and its properties. Wait, what is a bond? Carbon atoms primarily form covalent bonds with other carbon atoms. (Although fatty acid chains also contain Oxygen and Hydrogen). These covalent bonds are formed by the sharing of electron pairs between the carbon atoms, rather than a complete transfer of electrons (as in ionic bonding).
fatty acid chains

It’s all about the Chain. Fatty acids have different chain lengths and different compositions. Oils that have fatty acids with chain lengths between four and twenty-four carbons and most of them contain an even number of carbon atoms. When the carbon chain length is shorter, the melting point of the fatty acid becomes lower, and the fatty acid becomes more liquid.
Fatty acid chains are held together by carbon atoms that attach to each other and to hydrogen atoms. The term saturation refers to whether or not a fatty acid chain is filled (or “saturated”) to capacity with hydrogen atoms. If each available carbon bond holds a hydrogen atom, we call this a saturated fatty acid chain. All carbon atoms in such a fatty acid chain are bonded with single bonds. Sometimes the chain has a place where hydrogen atoms are missing. This is referred to as the point of unsaturation.
When one or more bonds between carbon atoms are a double bond (C=C), that fatty acid is called an unsaturated fatty acid, as it has one or more points of unsaturation. Any fatty acid that has only one double bond is a monounsaturated fatty acid, an example of which is olive oil (75 percent of its fat is monounsaturated). A polyunsaturated fatty acid is a fatty acid with two or more double bonds or two or more points of unsaturation. Soybean oil contains high amounts of polyunsaturated fatty acids. Both monounsaturated fats and polyunsaturated fats provide nutrition that is essential for normal cell development and healthy skin.
Oils and butters that have a high percentage of saturated fatty acids tend to be solid at room temperature. Coconut oil is a saturated fat. It contains approximately 90% saturated fatty acids, which are a type of fat that is solid at room temperature. It is primarily composed of saturated fats, particularly medium-chain fatty acids (MCFAs) like lauric acid. Oils rich in unsaturated fatty acids, such as olive oil (oleic acid, an eighteen-carbon unsaturated fatty acid, is a major component) tend to be liquid at room temperature. Flaxseed oil is rich in alpha-linolenic acid, which is an unsaturated fatty acid and becomes a thin liquid at room temperature.
Shea butter primarily contains long-chain fatty acids, specifically those with 18 carbon atoms. The main fatty acids in shea butter are stearic acid (C18:0) and oleic acid (C18:1), along with smaller amounts of linoleic acid (C18:2). The numbers refer to carbon molecules to the left of the colon, and number of double bonds to the right of the colon.
Castor oil is primarily composed of ricinoleic acid (18:1), a monounsaturated, 18-carbon fatty acid. This means the main fatty acid in castor oil has a chain length of 18 carbons. It’s unusual among fatty acids due to a hydroxyl group on the 12th carbon atom.
Short-chain fatty acids are found in plant sources like flax, chia, hemp, canola oil, and walnuts.
Medium Chain fatty acids like coconut oil, A primary source of MCTs, with varying concentrations of capric, caprylic, and lauric acids.
Palm kernel oil, another natural source of MCTs. Caproic acid (C6:0), caprylic acid (C8:0), capric acid (C10:0), lauric acid (C12:0). they are a type of saturated fats.
Long chain fatty acids include coconut oil, palm kernel oil, shea oil, cocoa butter, olive oil, soybean oil, and many other vegetable oils contain significant amounts of LCFAs.
LCFAs are also found in animal fats like butter, lard, and tallow, as well as in foods of animal origin like fatty fish (salmon, herring, trout). Avocado, nuts, and seeds are good sources of LCFAs. Avocados contain both saturated and unsaturated fats, approximately 75% of the fat in an avocado is considered good fat, including monounsaturated and polyunsaturated fats.
Knowing the connection between chain length, degree of saturation, and the state of the fatty acid (solid or liquid) is critical for the formulation of your soap recipe.
lye and pH
Lye is the compound sodium hydroxide (NaOH).
pH (potential for hydrogen) measures the concentration of hydrogen ions (H+) in a solution. A higher concentration of hydrogen ions means a solution is more acidic, and a lower concentration means it’s more basic (alkaline).
Lye functions as an alkali, reacting with fats or oils to produce soap and glycerin. The pH of sodium hydroxide is typically around 13-14.
The pH of soap typically falls within a basic range, usually between 8 and 10, with some soaps even reaching 11. This means soap is alkaline rather than neutral or acidic. The pH of soap can affect its suitability for different skin types, with more alkaline soaps potentially being harsher for sensitive skin. Different oils and fats used in soap making can affect the final pH as well as the amount and type of lye used to saponify the fats play a crucial role in determining the pH.
Now, Sodium hydroxide, also known as lye, needs to be dissolved in a liquid to dissociate into sodium ions (Na+) and hydroxide ions (OH-). Hydrogen ions (H⁺) and hydroxide ions (OH⁻) are fundamental to understanding acids and bases, particularly in aqueous solutions. The hydroxide ions are crucial for the saponification reaction to occur. They are the active agents that interact with the fats/oils to break them down into soap and glycerin. While water doesn’t appear directly in the final chemical equation of saponification, it acts as a solvent, releasing hydroxide ions from the compound NaOH allowing it to interact with the oils and fats effectively.
It’s also a good idea to mix your lye solutions beforehand, so they are at least room temperature the day of. This is because increasing temperature provides the reactant molecules with the necessary energy to collide effectively and drive the saponification reaction forward at a higher rate.
saponification
The process of making soap is a chemical reaction known as “saponification“. Soaponification would have been better… just saying…
Triglyceride (Fat/Oil) + Sodium Hydroxide (NaOH) → Glycerol + Soap (Sodium Salt of Fatty Acid)
Saponification involves the breaking of bonds within a single reactant (the triglyceride).
It is a decomposition reaction. It involves the breakdown of a polymer (triglyceride) into monomers glycerol and fatty acid salts (soap). The breakdown known as hydrolysis (water is added) occurs with a strong base like sodium hydroxide breaking the ester bonds in the triglyceride.
As I understand it, soap is a completely different compound created from a hydrolysis chemical reaction. This is NOT an emulsion of oil and water. Although, the end product of the reaction, which is soap, acts as an emulsifier of water and oil by acting as a surfactant.
Now I know what you’re thinking…
Emulsifiers are substances that allow oil and water to mix and remain mixed, and sodium hydroxide’s role in soap making is to break down fats and oils, not to stabilize them in an emulsion.
Emulsification is not a part of the saponification reaction itself, but it is a key mechanism through which the soap produced by saponification performs its cleaning function.
The fatty acids created during saponification act as surfactants, which allow oil and water to mix.
how does soap clean and disinfect?
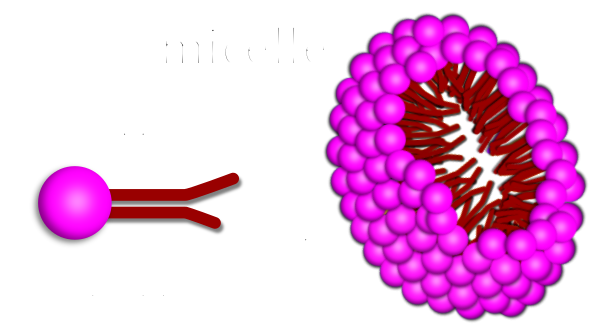
A micelle is a wheel-like structure formed by the circle of soap molecules around a dirt or oil droplet. The molecules of soap of sodium salts are made of long chain carboxylic acids. The ionic end of the salts of soap dissolves in the water of the soap solution, while the carbon chain dissolves in oil which is present in the dirt, forming structures called micelles. Micelles are formed by amphiphilic molecules which are present in the salt of the soap. The structures of the micelles contain hydrophilic (water loving) and hydrophobic (water hating) ends. They surround dirt and float away in the water rinse. They break down dirt and pathogens.
Here’s how soap micelles break down pathogens:
Soap molecule structure: Each soap molecule has a hydrophilic (water loving) head and a hydrophobic (water hating) tail.
Interaction with fats and oils: The hydrophobic tail of the soap molecule is attracted to fats and oils. Many bacteria and viruses, including coronaviruses, are enclosed within a fatty lipid membrane.
Disruption of lipid membranes: When soap encounters these pathogens, the hydrophobic tails of the soap molecules wedge themselves into the lipid membranes of the bacteria and viruses, effectively prying them apart.
Micelle formation: As the soap molecules interact with the lipids, they form micelles, which are tiny spherical clusters. The hydrophobic tails of the soap molecules surround the fragments of the disrupted lipid membrane, with the hydrophilic heads facing outwards towards the water.
Encapsulation and removal: The formed micelles encapsulate the disrupted lipid membrane fragments and any associated genetic material or proteins, making them soluble in water.
Washing away: When you rinse with water, the hydrophilic heads of the micelles are attracted to the water molecules, and the entire structure, including the trapped pathogen components, is washed away.
Essentially, the soap micelles act as molecular “crowbars”, destabilizing the lipid membranes of the pathogens and then encapsulating the resulting fragments so they can be effectively rinsed away with water. This action inactivates the pathogens and removes them from surfaces.
Soap molecules dislodge dirt, oil, and grease particles, and the disease-causing germs they carry, from your hands, one micelle at a time.
finally
So that was a lot to digest! Now you know how our stomachs feel…
All of the above being said, how does this effect the soap making process? The amount of water in a cold process soap recipe significantly affects the soap’s characteristics. Water quantity influences trace time, gel phase, saponification, and ultimately, the final soap’s hardness, cure time, and appearance.
The temperature of your lye solution and oils will greatly effect the speed of the “trace”. Lye and oil being an exothermic reaction means that the hotter your lye solution (and oils) the quicker the reaction. Higher temperatures will shift the equilibrium of exothermic reactions towards the reactants, favoring their formation. This is because increasing the temperature adds heat, which is considered a product in exothermic reactions. According to Le Chatelier’s principle, the system will counteract this stress by favoring the reverse reaction, consuming the added heat and shifting the equilibrium to the left. In the saponification reaction, the primary reactants are fats or oils (triglycerides) and a strong base, typically sodium or potassium hydroxide (lye). These reactants are also sometimes referred to as “esters” in organic chemistry. The reaction also requires water for the hydrolysis process.
Some oils have longer fatty acid chains (shea butter, coconut oil) and therefore have a higher melting point (the temperature at which time they become liquid). Some oils have shorter fatty acid chains (olive oil, cator oil) and therefore are liquid at much lower temperatures. Knowing this will help you create a recipe that you can keep in a state that is workable for soap molding.
Since we’re talking so much science, though it is true that water in its solid form (ice) will increase by about 9% volume, the state’s mass is still the same. In other words, 1 gram of water is one gram of water whether frozen, liquid, or steam.